
The olefin metathesis reaction (the subject of 2005 Nobel Prize in Chemistry) can be thought of as a reaction in which all the carbon-carbon double bonds in an olefin (alkene) are cut and then rearranged in a statistical fashion:
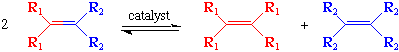
If one of the product alkenes is volatile (such as ethylene) or easily removed, then the reaction shown above can be driven completely to the right. Likewise, using a high pressure of ethylene, internal olefins can be converted to terminal olefins. There are a wide variety of variants on this reaction as is discussed below.
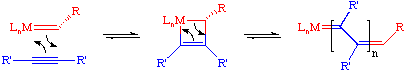
The commonly accepted mechanism for the olefin metathesis reaction was proposed by Chauvin and involves a [2+2] cycloaddition reaction between a transition metal alkylidene complex and the olefin to form an intermediate metallacyclobutane. This metallacycle then breaks up in the opposite fashion to afford a new alkylidene and new olefin. If this process is repeated enough, eventually an equilibrium mixture of olefins will be obtained.
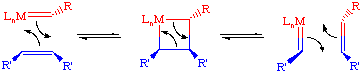
Such cycloaddition reactions between two alkenes to give cyclobutanes is symmetry forbidden and occurs only photochemically. However, the presence of d-orbitals on the metal alkylidene fragment breaks this symmetry and the reaction is quite facile.
Chemical & Engineering News had a good article called Olefin Methathesis: The Early Days that discusses the early discoveries in the field and the elucidation of the mechanism [Chem. & Eng. News 2002, Dec 23, 34-38].
> There have been roughly four distinct generations of olefin metathesis catalysts:
While these catalysts are exceedingly active, they have an exceedingly low tolerance for functional groups because of their Lewis acidic nature. Likewise, less than one percent of the material is an active catalyst, and nothing is known about the nature of the actual catalytic species in these systems. One commercial application still using these catalysts is the ROMP of dicyclopentadiene to produce tough plastics for use in golf carts, snow mobile hoods etc.
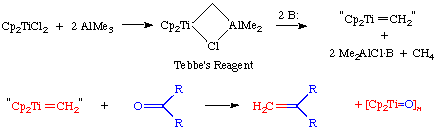
These Ti-based catalysts are not nearly as active or tolerant of carbonyl functionalities as the later catalysts, but Grubbs has shown that these Ti complexes undergo stoichiometric Wittig-like reactions with ketones, aldehydes and other carbonyls to form the corresponding methylene derivatives. The mechanism of this reaction is identical to that of the olefin metathesis reaction except that the final step is not reversible.
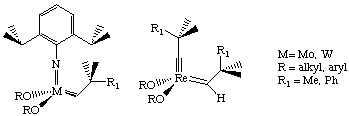
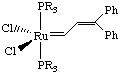
These catalysts have a high tolerance for functionality, although they are air and water-sensitive. Two important features of these catalysts are that they are 100% active and have been fully characterized by NMR and X-ray crystallography. The success of these catalysts stems from their coordinative and electronic unsaturation (making them electrophilic) and their bulky ligands (prevents bimolecular decomposition).
The ADMET method, pioneered by Ken Wagener and Jim Boncella at the U of Florida, uses alpha-omega dienes to produce polymers. The reaction is driven by the removal of ethylene from the system, which can be accomplished with a nitrogen purge.
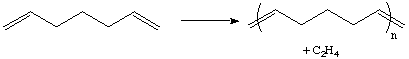
The reverse of this reaction (reacting an unsaturated polymer with excess ethylene in the presence of a metathesis catalyst), has been studied as a possible means of recycling automobile tires. However, given the challenge of finding a highly active catalyst that can tolerate the functional groups in tires (sulfur, carbon black etc.), it remains to be seen if this will method will become commercially viable.
When an acetylene is reacted with an alkylidene, a [2 + 2] cycloaddition occurs as with olefins, a metallacyclobutene is formed instead of a metallacyclobutane. If this metallacycle opens in a productive fashion, the result is a growing polymer chain:
This reaction typically only works well with 2-butyne or terminal acetylenes. Polymerization of terminal acetylenes is complicated by the potential for the R group to insert alpha or beta with respect to the metal. It is extremely challenging to always get a beta insertion and generate a polymer with reproducible properties.