The acetylene metathesis reaction is closely related to the olefin metathesis reaction. The acetylene metathesis reaction can be thought of as one in which all the carbon-carbon triple bonds in a mixture of acetylenes (alkynes) are cut and then rearranged in a statistical fashion:
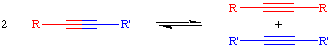
Acetylene metathesis is not commonly performed and is not used in industry for two primary reasons. First, acetylenes are easily and cheaply made using other methods. Second, competing side reactions (examples below) are unavoidable.
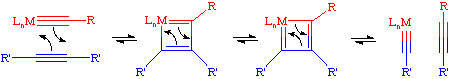
The commonly accepted mechanism for the acetylene metathesis reaction parallels the Chauvin mechanism for olefin metathesis. A [2+2] cycloaddition reaction between a transition metal alkylidyne complex and the acetylene leads to an intermediate metallacyclobutadiene. This metallacycle then breaks up in the opposite fashion to afford a new alkylidyne and a new acetylene. If this process is repeated enough times, eventually an equilibrium mixture of acetylenes will be obtained.
It should be noted here that reaction of acetylenes with alkylidenes leads to the formation of conjugated polymers.
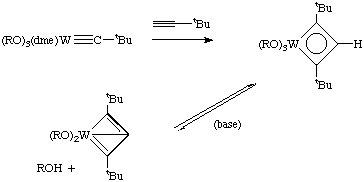
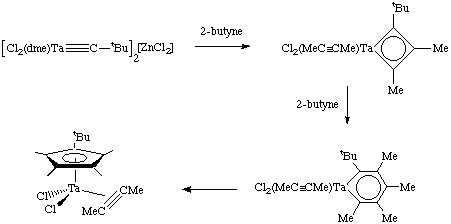
Schrock has reported several catalytic systems for acetylene metathesis, but competing side reactions are still a major problem. Here are just two examples: