
The word agostic is derived from the Greek word for "to hold on to oneself". It most commonly refers to a C-H bond on a ligand that undergoes an interaction with the metal complex. This interaction closely resembles the transition state of an oxidative addition or reductive elimination reaction. The bonding could also be described as closely resembling a sigma complex of an alkane or silane or as resembling a dihydrogen complex in which H2 acts as a two electron sigma donor.
Three examples of agostic interactions are shown below. Compound B, synthesized by Al Cotton and coworkers in 1974, was the first example of an agostic interaction. In compound C the agostic structure is much more stable than either the alkene-hydride or the alkyl.
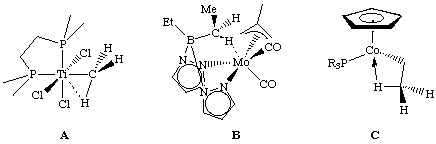
Agostic interactions can be detected by the presence of a 1H NMR peak that is shifted from that of a normal aryl or alkane towards that of a hydride ligand (which are typically -5 to -15 ppm, but can be in the range of +25 to -60 ppm). Moreover, the JCH is typically around 70-100 Hz versus the 125 Hz expected for a normal sp3 carbon atom. These complexes are usually fluxional which complicates the analysis. A reduced C-H stretching frequency can sometimes be observed in the IR.
Structural information is best gained from neutron diffraction data given the difficulty that exists in pinpointing hydrogen atom positions by X-ray diffraction. Typical M-H distances in agostic complexes are about 1.85 to 2.4 angstroms.
As an example of these features, consider compound B above. The M-H distance was found to be 2.1 angstroms, IR bands were observed at 2704 and 2664 cm-1 and the agostic proton was observed at -3.8 ppm. The two hydrogens on the agostic methylene are rapidly switching between terminal and agostic on the NMR time scale.