
As the name implies reductive elimination involves the elimination or expulsion of a molecule from a transition metal complex. In the process of this elimination, the metal center is reduced by two electrons. In the simplest example below the metal goes from the x+2 to the x oxidation state and a coordinatively unsaturated metal center is obtained. In Equation 2 we see a case of a binuclear reductive elimination reaction:
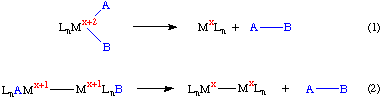
Reductive elimination is formally the microscopic reverse of oxidative addition, and it is not surprising that a series of reactions involving an oxidative addition, a rearrangement and then a reductive elimination form the basis for a variety of industrially important catalytic cycles.
Important principles to remember about reductive elimination are:
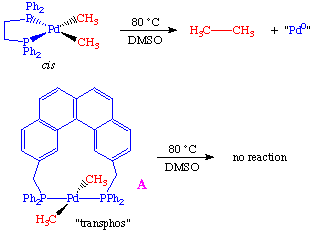
To rule out the possibility that the transphos ligand was simply changing the chemistry, the authors performed a crossover experiment which further supported the hypothesis of a mutually cis requirement:
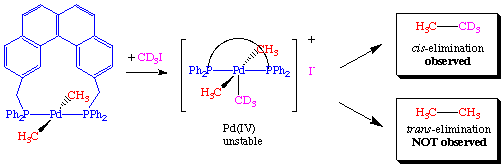
This latter reaction is also an example of an oxidatively induced reductive elimination (see below).
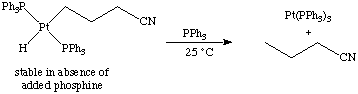
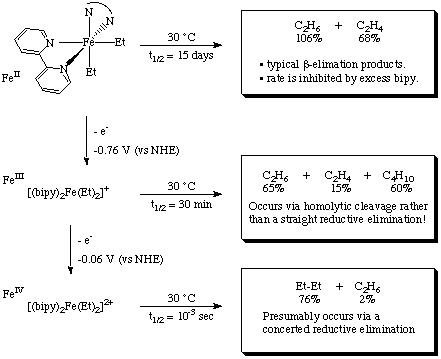
Notice that three different decomposition mechanisms are operative for the three different oxidation states of iron!
The rates of reductive elimination (as well as oxidative addition) depend on a variety of factors, but one of the most important is thermodynamics. Consider the following examples:
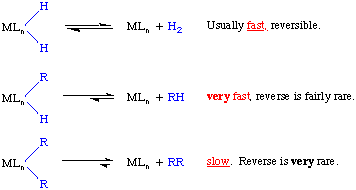
If we consider that the DH-H = 104 kcal/mol and that the DM-H is 50-60 kcal/mol we see that these are essentially balanced and there should be no thermodynamic preference for a dihydride versus a reduced metal center.
But DR-H is typically 100 kcal/mol versus a metal alkyl bond strength of 30 to 40 kcal/mol. We see that the thermodynamic situation is again approximately balanced with a slight preference for the forward reaction.
DR-R is typically around 90 kcal/mol, so for two alkyl substituents, there is a strong thermodynamic driving force for the reaction to go to the right. C-C bond activation is unusually rare, but more examples continue to be found.
| If you like the self-test exercises presented here, give me some feedback via email. If there is enough support, I'll add more throughout the OMHTB. - RT |
THESE QUIZZES ARE NOT CURRENTLY WORKING - We moved to a new server platform in September 2025 and I have to go back and redo the coding that drives the grading. Stay tuned...