



Find all of your laboratory and workplace safety supplies at Safety Emporium!
 NIOSH |
 Glossary Index |
 NOS |
| MSDS Topics |
Free Sites | FAQ's | Regulations | Glossary | Software | Suppliers |
| Books | Forum | Poll | Fun stuff | Quiz | Store | |
| Understand your MSDS with the MS-Demystifier | Search ALL our MSDS info | |||||

Get your GHS-compliant labels and signs from Safety Emporium.
A nitrile is an organic chemical that contains a cyano functional group (subunit), CN-, in which the carbon and nitrogen atoms have a triple bond i.e. C≡N-. The general chemical formula of a nitrile is RCN, where R is the organic group.
Nitriles can be thought of as organic cyanides, although they are ordinarily much less toxic than simple cyanide salts such as sodium or potassium cyanide (NaCN or KCN).
Under the standard chemical naming convention used by chemists (IUPAC nomenclature), nitriles are named according to these rules:
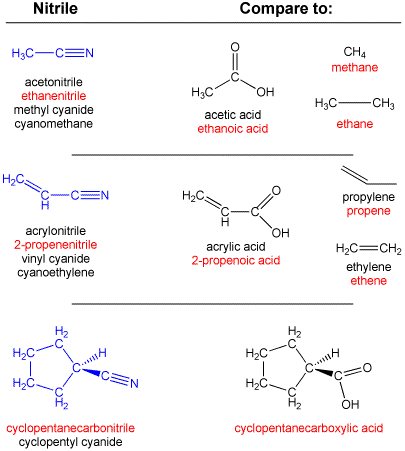
In addition, the use of cyanide as a suffix is still common, and one nitrile may be known by a variety of different names. Some examples of these are shown below, with the most rigorous IUPAC name for each shown in red:
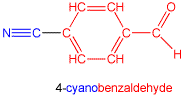
When there are other functional groups that take naming precedence over the cyanide/nitrile group, the prefix cyano is used. In this example, we have color-coded the parts of the name and the parts of the molecule that correspond to each other:
Nitriles are usually less toxic than cyanide salts, although they may contain some of the latter as impurities. Aliphatic nitriles can be metabolized to the free cyanide ion, making them generally more toxic than arylnitriles which are stable in the body.

When you work with chemicals, eye washes, safety showers and equipment signs are a must. Get yours at Safety Emporium.
The cyanide unit occurs in other compounds such as cyanamide (carbodiimide, H2NC≡N), cyanoacetic acid (malonic mononitrile, N≡CH2COOH), potassium ferricyanide (K3[Fe(CN)6]) and potassium ferrocyanide (K4[Fe(CN)6]), however these compounds do not release cyanide and are therefore much less toxic than simple cyanide salts or many nitriles.
Nitriles have tremendous industrial importance. For example, through a process called hydrocyanation, HCN reacts with 1,4-butadiene (an alkene) to form adiponitrile (also referred to as 1,4-dicyanobutane, hexanedinitrile, tetramethylene cyanide, or the formula NC(CH2)4CN), a chemical precursor to hexamethylene diamine (also known by 1,6-diaminohexane or the formula H2N(CH2)6NH2), one of the polymers used to make nylon. The wide range of chemical reactivity for nitriles is what makes them so useful, but this same feature means that they are incompatible with many substances (see SDS relevance below).
Nitriles are also important laboratory and industrial solvents because of their unusual physical properties. For example, nitriles boil much higher than the corresponding hydrocarbon of similar molecular weight; acetonitrile (CH3CN, FW 41 amu) boils at 81 °C whereas propane (CH3CH2CH3, FW 44 amu) boils at -42 °C. This increase can be ascribed to the polar nature (uneven distribution of electron density) of nitriles; the C≡N bond is polarized and intermolecular alignment of these dipoles increases the intermolecular attraction between the solvent molecules. In the drawing below, the lone pair of electrons on the nitrogen atom (present in all nitriles, but normally omitted from simple drawings) is explicitly shown to help the reader understand the polarity of the molecule:

This polarity makes nitriles terrific aprotic (no easily ionized hydrogen atom) solvents. For example, acetonitrile is completely miscible with water and methanol as well as organic solvents such as diethyl ether, ethyl acetate, and acetone; at the same time it is virtually insoluble in saturated hydrocarbons. This versatility makes it trivially easily to remove acetonitrile from reaction mixtures without resorting to distillation. Its high dielectric constant (38) and dipole moment (3.9 Debye) make acetonitrile ideal for promoting chemical reactions where ionization is involved, as a solvent for inorganic salts (electrolytes), and as a medium for electrochemical studies.

Your employees can stay informed and comply with OSHA regulations with SDS information stations and compliance products from Safety Emporium.
Nitriles usually appear on Safety Data Sheets in the context of incompatible materials in Section 10 (stability and reactivity) of the SDS. In general, nitriles are incompatible with acids, bases, amines, oxiranes, and acid anhydrides.
Nitriles can produce other nitriles and highly toxic HCN gas as well as carbon monoxide when burned. You may therefore also see nitriles mentioned as a toxic byproduct of combustion under Section 5 (fire-fighting measures) or Section 10 (stability and reactivity) of the SDS.
Although not highly toxic like inorganic cyanides or hydrogen cyanide, nitriles are generally toxic materials and should be used with proper engineering controls and personal protective equipment as recommended in Section 8 (exposure controls/personal protection) of the SDS. As with any hazardous substance, minimize your exposure or potential exposure.
Nitrile may also appear on an SDS as a recommended type of glove, boot, apron or other piece of personal protective equipment. Polymerization of acrylonitrile gives nitrile rubber, a substance with excellent resistance to oils and greases. Nitrile gloves are low cost, have good physical properties, and offer good dexterity. However, they are a poor choice for working with benzene, methylene chloride, trichloroethylene, and many ketones. In this context, the compatibility of the substance with respect to nitrile gaskets, seals or o-rings may also be mentioned.
See also: amine, carboxylic acid, cyanide, cyanosis, volatility.
Additional definitions from Google and OneLook.
Entry last updated: Friday, January 6, 2023. This page is copyright 2000-2025 by ILPI. Unauthorized duplication or posting on other web sites is expressly prohibited. Send suggestions, comments, and new entry desires (include the URL if applicable) to us by email.
Disclaimer: The information contained herein is believed to be true and accurate, however ILPI makes no guarantees concerning the veracity of any statement. Use of any information on this page is at the reader's own risk. ILPI strongly encourages the reader to consult the appropriate local, state and federal agencies concerning the matters discussed herein.