



Find all of your laboratory and workplace safety supplies at Safety Emporium!
 Ames Test |
 Glossary Index |
 Analgesia |
| MSDS Topics |
Free Sites | FAQ's | Regulations | Glossary | Software | Suppliers |
| Books | Forum | Poll | Fun stuff | Quiz | Store | |
| Understand your MSDS with the MS-Demystifier | Search ALL our MSDS info | |||||
Filter mixtures at constant temperature with laboratory frits and filters from Safety Emporium.
Amine molecules have the general formula of R3-xNHx where R is a hydrocarbon group, and x is an integer with 0 < x < 3. Put another way, amines are derivatives of ammonia, NH3, in which one or more hydrogen atoms have been replaced by hydrocarbon groups. Specific examples of amines are shown in the next section.
An amino group refers to an NH2 sub-unit within a molecule. See Additional Info below.
Amines are part of the basic building blocks of life, amino acids, and are widely found in Nature. Because of their chemical reactivity, high availability, and ease of handling, they have widespread use in materials that we use every day.
The nitrogen atom in an amine molecule has a lone pair of electrons which makes most amines act as weak bases. Therefore, most amines react readily with acids, and solutions of amines have pH's greater than 7 (alkaline, basic).
Many amines are volatile and have distinctive, nasty, pungent odors; keep reading!
Some amines and types of amines that merit additional discussion are:
Ammonia is the simplest possible amine. The molecule has a trigonal pyramidal structure as shown below. The lone pair of electrons points up from the top of this pyramid; if this lone pair were a hydrogen atom we could call the shape tetrahedral:
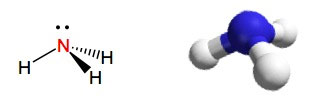
Ammonia has a boiling point of -33 degrees C and is therefore a gas at room temperature. Although ammonia is a weak base, it is corrosive and can cause severe lung damage or death when inhaled in large concentrations.
Solutions of ammonia in water are called aqueous ammonia. A solution of 28-29% of ammonia in water is sometimes called ammonium hydroxide and given the chemical formula NH4OH (such molecules do not actually exist, but this is an old chemist's habit that refuses to die). 10% solutions of ammonia in water are used as "smelling salts".
Household ammonia solutions are typically more dilute. Mixing household ammonia and chlorine bleach is exceedingly dangerous and produces a toxic and carcinogenic mixture of chloramine, NH2Cl, and hydrazine, N2H4 (not, as many believe, chlorine gas, Cl2).
Primary amines are derivatives of ammonia in which one hydrogen has been replaced by a hydrocarbon unit. Some common primary amines (and 3 different ways of naming each) are shown here:
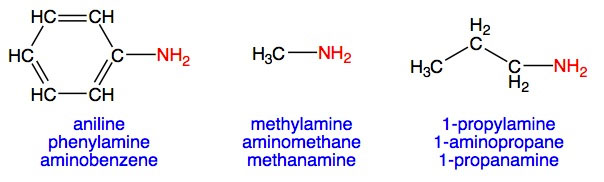
Aniline, shown above, is an example of an industrially important aromatic amine.

Hold on to your reaction with laboratory clamps and accessories from Safety Emporium.
Secondary amines are derivatives of ammonia in which two hydrogens have been replaced by hydrocarbon units. These two units can be the same or different:
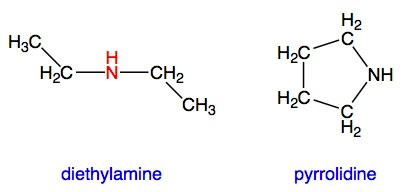
Secondary amines are usually less reactive than primary amines because the hydrocarbon groups are larger than a hydrogen atom. This extra bulk reduces the ability of an incoming reactant molecule to interact with the nitrogen atom.
Tertiary atoms have three hydrocarbon substituents which may be the same or different. Some examples include:
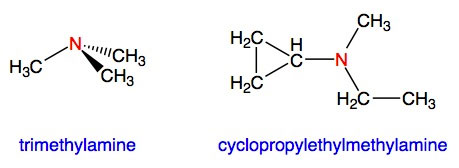
Trimethylamine is a gas with a strongly fish-like odor. In fact, together with other amines, trimethylamine is responsible for the smell of rotting fish.
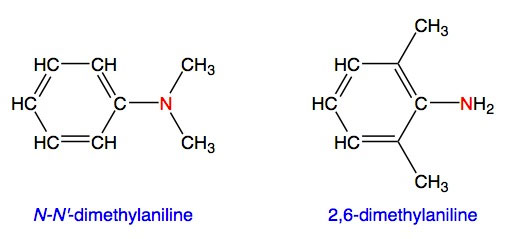
To avoid confusion in naming amines, the substituents (groups) that are attached to the nitrogen atom are given the prefixes N and N'. For example, both N,N'-dimethylaniline and 2,6-dimethylaniline have two methyl (CH3) groups, but they are tertiary and primary amines, respectively:

Get your corrosion-resistant polyethylene storage cabinets with internal sumps from Safety Emporium.
Amines can react with acids and other chemical groups to form quaternary ammonium salts. For example, reaction of ammonia, NH3, with hydrogen chloride, HCl, gives the salt, ammonium chloride, NH4Cl which consists of ammonium cations, NH4+, and chlorine anions, Cl-. Another example is tetramethylammonium bromide, shown here:
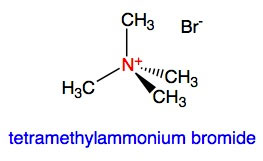
Because quaternary ammonium salts are ionic species, they are good electrolytes. Such salts are often used in detergents and other cleaning agents. Quaternary amine salts may produce acidic, basic or neutral solutions depending on the nature of the R groups and the anion (negatively charged ion).
Amines have widespread uses in industry. Unfortunately, they are incompatible with many substances, particularly acids. These hazards will typically be noted in Section 10 (stability and reactivity) of the Safety Data Sheet.
Many amines are poisonous by inhalation, ingestion or skin absorption. Always use the proper proper personal protective equipment (PPE) mentioned on the SDS such as gloves, goggles and aprons. Reducing your use of amines in the workplace, or utilizing fume hoods may help reduce hazards.
Many amines are somewhat soluble in water (and most ammonium salts are), so be sure that any aqueous wastes you dispose of down the drain meet the appropriate EPA guidelines.

Your chemical reactions can be run safely and effectively with US-made clamps and other laboratory accessories from Safety Emporium.
Additional definitions from Google and Onelook.
Entry last updated: Monday, February 21, 2022. This page is copyright 2000-2025 by ILPI. Unauthorized duplication or posting on other web sites is expressly prohibited. Send suggestions, comments, and new entry desires (include the URL if applicable) to us by email.
Disclaimer: The information contained herein is believed to be true and accurate, however ILPI makes no guarantees concerning the veracity of any statement. Use of any information on this page is at the reader's own risk. ILPI strongly encourages the reader to consult the appropriate local, state and federal agencies concerning the matters discussed herein.