



Find all of your laboratory and workplace safety supplies at Safety Emporium!
 H-statements |
 Glossary Index |
 Hygroscopic |
| MSDS Topics |
Free Sites | FAQ's | Regulations | Glossary | Software | Suppliers |
| Books | Forum | Poll | Fun stuff | Quiz | Store | |
| Understand your MSDS with the MS-Demystifier | Search ALL our MSDS info | |||||

Safety Emporium has a great lineup of gas cylinder signs, storage racks, lockouts, clamps and more.
A hydrocarbon is an organic chemical compound that is comprised only of carbon (C) and hydrogen (H) atoms.
Many oils, fats, waxes, solvents and paraffin are either hydrocarbons or contain large hydrocarbon sub-units.
Hydrocarbons can be divided into particular classes depending on their properties. Some examples are:
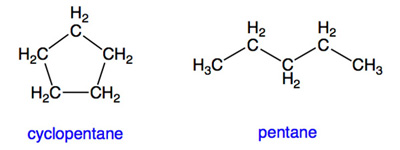
Hydrocarbons (or other organic molecules) that form a ring are called cyclic. Those that do not have rings are called acyclic or non-cyclic. For example, cyclopentane (C5H10) is cyclic, while pentane (C5H12) is acyclic:
Note: The terms "saturated" and "unsaturated" also refer to the concentration of solutions. These have a much different meaning and context.
A saturated hydrocarbon (or other organic molecule) has utilized all of its bonding electrons to make single bonds to other atoms. It can not make additional bonds without removing an existing bond to another atom or group of atoms in the molecule.
An unsaturated hydrocarbon (or other organic molecule) contains double or triple bonds between certain atoms. These bonds may be broken and new atoms or groups of atoms attached without disrupting the existing skeleton of the hydrocarbon.
If a molecule contains a saturated hydrocarbon sub-unit, this sub-unit is called an alkyl group. If the sub-unit is an aromatic group this sub-unit is called an aryl group.
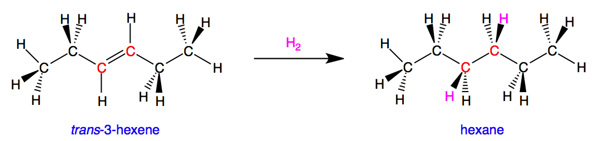
For carbon, the maximum (and ideal) number of bonds is four. Let's look at an example. trans-3-hexene is unsaturated because the atoms shown in red have two bonds connecting them and are bound to only three different atoms. If we chemically add hydrogen atoms to the red carbons, this reduces the carbon-carbon double bond to a single bond and gives the red carbons four single bonds to other atoms. The resulting molecule, hexane (also called n-hexane), is saturated because we can not add any more atoms to it without removing bonds to existing atoms or groups of atoms.
You may have heard the terms monounsaturated, polyunsaturated, and saturated in reference to fats and oils. Saturated fats (no double bonds) contain long hydrocarbon sub-units (alkyl groups). Examples are coconut oil and lard, which have a tendency to clog your arteries because of their chemical and physical properties. Unsaturated fats, those with one (mono) or many (poly) double bonds, are less likely to form artery-clogging plaques.
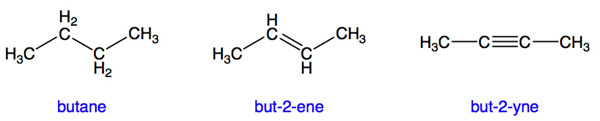
The following prefixes are commonly used for simple hydrocarbons, but are not part of the systematic naming scheme used by chemists. For systematic naming rules (vital to name a hydrocarbon with more than one branch!), see the IUPAC link below.
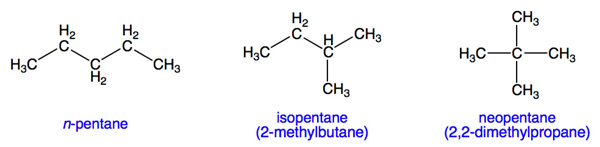
The molecules shown above have the same chemical formula (C5H12), but a different arrangement in each case, so we call them isomers. These isomers of pentane have different chemical reactivity and properties because of their different molecular arrangements. For example n-hexane is a neurotoxin while neohexane is not.

Be compliant with confined space products from Safety Emporium.
Pay close attention to the name of the chemical with which you're working so you don't end up consulting the wrong Safety Data Sheet. As noted above, pentane and isopentane are two completely different chemicals.
Hydrocarbons and their chemical derivatives are often quite flammable. Many of these are toxic or present other health hazards. If possible, substitute a less hazardous solvent.
Always wear proper gloves and eye protection when working with hydrocarbons or organic chemicals. Be sure to wear an appropriate respirator if ventilation is not adequate, and be careful to avoid ignition sources as the vapors can easily reach their flammable limit. When dispensing bulk quantities of flammable liquids, proper bonding and grounding procedures must be followed to avoid the formation of static electricity which could ignite the vapors.
"Empty" hydrocarbon tanks pose two specific dangers. First, if it is a large tank, the heavier-than air vapors can create an oxygen-deficient atmosphere that is immediately dangerous to life and health. Proper confined space entry procedures should be developed and used for any tank large enough to be entered. Second, there are dozens of examples where workers have been killed or injured when they accidentally ignited residual vapors in an "empty" tank by welding, cutting, or grinding. See the links below for examples and precautions.

Hold on to your reaction with laboratory clamps and accessories from Safety Emporium.
See also: aliphatic, aromatic, flammable limits, organic.
Additional definitions from Google and OneLook.
Entry last updated: Sunday, January 1, 2023. This page is copyright 2000-2025 by ILPI. Unauthorized duplication or posting on other web sites is expressly prohibited. Send suggestions, comments, and new entry desires (include the URL if applicable) to us by email.
Disclaimer: The information contained herein is believed to be true and accurate, however ILPI makes no guarantees concerning the veracity of any statement. Use of any information on this page is at the reader's own risk. ILPI strongly encourages the reader to consult the appropriate local, state and federal agencies concerning the matters discussed herein.