



Find all of your laboratory and workplace safety supplies at Safety Emporium!
 Chemical |
 Glossary Index |
 CHIP |
| MSDS Topics |
Free Sites | FAQ's | Regulations | Glossary | Software | Suppliers |
| Books | Forum | Poll | Fun stuff | Quiz | Store | |
| Understand your MSDS with the MS-Demystifier | Search ALL our MSDS info | |||||

Ensure a safe workplace with antislip floor marking signs from Safety Emporium.
A chemical formula expresses the exact composition of a molecule or substance using the chemical abbreviations of the chemical elements.
A chemical element is a substance that can not be separated into simpler substances by chemical means. There are currently 118 known chemical elements of which 94 are naturally occurring (albeit some of those occur only in minuscule quantities). Common examples of chemical elements include carbon, hydrogen, chlorine, sodium and iron.
The smallest basic unit of a chemical element that can enter into chemical reactions is called an atom.
There are several different ways of expressing chemical formulas:
Molecular formulas use the exact number of atoms of each element present in the smallest unit of the substance. For example, benzene is a molecule composed of six carbon and six hydrogen atoms and has a formula C6H6.
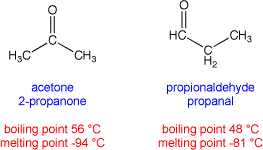
Note: two different materials may share the same molecular formula. For example, both acetone (also called 2-propanone) and propionaldehyde (also called propanal) share the same molecular formula of C3H6O, but have different arrangements of atoms in the molecules. These are called structural isomers. Structural isomers usually have different chemical and physical properties:
Empirical formulas use the simplest (lowest) whole-number ratio of the elements that are present. For example, the molecular formula of benzene is C6H6, but the empirical formula is simply CH.
Note: An even larger number of compounds can share the same empirical formula. For example, acetylene (a gas) has the molecular formula C2H2, but has the same empirical formula as benzene (a liquid), CH.
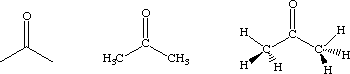
Structural formulas are a version of a molecular formulas in which the connectivity of the atoms is implied. For example, acetone, which has a molecular formula of C3H6O, can also be expressed as (CH3)CO(CH3) or (CH3)2CO. For convenience, chemists often sketch these. Those shown below for acetone all express the same structure:
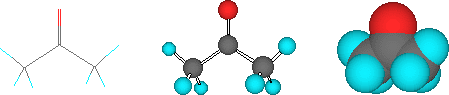
Finally, while you probably won't see these on SDS's, chemists sometimes use structural models to represent the arrangement of atoms in a molecule (the molecular stucture). Again, these are all equivalent ways of drawing the structure of acetone:

Safety Emporium has all kinds of lab equipment such as this incubating rocking shakers and more.
Safety Data Sheets provide key information about the chemicals that you use in your workplace. Be sure to read and understand the SDS of every substance you use or are exposed to at work before you work with the material.
Recognize that a molecular formula is not necessarily a unique identifier for a chemical substance. Do not rely on molecular formulas alone to label or identify a substance. For example, the molecular formulas of glucose (a form of sugar) and 1,3-dihydroxy-2-propanone dimer (an eye and skin irritant) are identical, C6H12O6.
Chemical names are not always reliable because a common chemical may be known by several different names. For example, methylene chloride, dichloromethane and methylenebichloride are common names for the same substance, CH2Cl2. If you need a unique identifier, use the CAS Registry Number as well as molecular formula and name.
See also: CAS Registry Number, mole.
Additional definitions from Google and OneLook.
Entry last updated: Sunday, July 3, 2022. This page is copyright 2000-2025 by ILPI. Unauthorized duplication or posting on other web sites is expressly prohibited. Send suggestions, comments, and new entry desires (include the URL if applicable) to us by email.
Disclaimer: The information contained herein is believed to be true and accurate, however ILPI makes no guarantees concerning the veracity of any statement. Use of any information on this page is at the reader's own risk. ILPI strongly encourages the reader to consult the appropriate local, state and federal agencies concerning the matters discussed herein.