



Find all of your laboratory and workplace safety supplies at Safety Emporium!
 Evaporation rate |
 Glossary Index |
 Explosive |
| MSDS Topics |
Free Sites | FAQ's | Regulations | Glossary | Software | Suppliers |
| Books | Forum | Poll | Fun stuff | Quiz | Store | |
| Understand your MSDS with the MS-Demystifier | Search ALL our MSDS info | |||||

Store and dispense flammable liquids safely with flammable liquid safety cans from Safety Emporium.
An exothermic process is one that gives off heat. This heat is transferred to the surroundings.
An endothermic process is one in which heat has to be supplied to the system from the surroundings.
A thermoneutral process is one that neither requires heat from the surroundings nor gives off energy to the surroundings.
These terms are usually applied to chemical reactions. A chemical reaction can only be one of these three terms at any given moment. A reaction that is exothermic will be endothermic if run backward and vice-versa.
Exothermic chemical reactions liberate heat. A simple and familiar example is the combustion of methane gas (CH4). The balanced chemical reaction for this process is:
CH4(g) + 2 O2 (g) 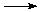 CO2(g) + 2 H2O(g).
CO2(g) + 2 H2O(g).
This process gives off a lot of energy, so we could write "heat" as one of the products on the right (products) side of the reaction if we wished. The term enthalpy, H, is used by chemists to describe how heat flows into or out of a system.
For an exothermic reaction, the change in enthalpy, ΔH, as we go from reactants (methane and oxygen) to products (carbon dioxide and water) is a negative quantity. For an endothermic reaction, ΔH is greater than zero. And for a thermoneutral reaction ΔH = 0.
ΔH tells us how much heat energy will be given off or will be required for a particular chemical reaction. It doesn't tell us whether the reaction will occur or how fast. A great example of strongly exothermic reaction that does not occur without a significant heat input to get it started is the thermite reaction which is so energetic that it can weld metal under water!
Endothermic reactions are usually not a great safety hazard. However, because the reaction draws heat from its surroundings, the reaction container may become cold and cause condensation or ice to form. This can be a safety hazard if the materials involved react with water.

Get your Class D and other specialty extinguishers from Safety Emporium.
These terms are not commonly found on Safety Data Sheets. If mentioned, exothermic is usually used in describing a sudden release of a large amount of heat in either Section 5 (fire-fighting measures), Section 6 (accidental release measures), or possibly Section 10 (stability and reactivity). Endothermic may be found similarly, but endothermic reactions are far less common.
Many common chemicals undergo exothermic reactions. For example, simply dissolving sodium hydroxide (NaOH) in water produces enough heat that if this is not done carefully it could melt a plastic container! Most mixtures of incompatible chemicals involve violently rapid exothermic reactions.
The heat that a chemical reaction gives off can quickly heat the surrounding area (or rest of the chemicals in the container) to very high temperature. As temperature increases, the rate of chemical reactions generally increase as well. Thus, once an exothermic reaction begins, it can quickly "run away" - accelerating its rate because of the heat produced. This can be especially dangerous if the material reaches its flash point or autoignition temperature, at which point a fire or explosion could occur. Therefore, always conduct a thorough risk assessment before performing (and especially before scaling up) an exothermic reaction.
Again, it is critically important to know when a chemical reaction can generate excess heat and to take appropriate measures to deal with this. Examples include slow mixing, using a cooling bath, or avoiding that reaction. When performing scaleups, be sure you carefully monitor the temperature, do not scale up too much in one leap, and prepare ahead of time for possible emergencies.
See also: autoignition temperature, flash point, oxidation.
Additional definitions from Google and OneLook.
Entry last updated: Sunday, July 24, 2022. This page is copyright 2000-2025 by ILPI. Unauthorized duplication or posting on other web sites is expressly prohibited. Send suggestions, comments, and new entry desires (include the URL if applicable) to us by email.
Disclaimer: The information contained herein is believed to be true and accurate, however ILPI makes no guarantees concerning the veracity of any statement. Use of any information on this page is at the reader's own risk. ILPI strongly encourages the reader to consult the appropriate local, state and federal agencies concerning the matters discussed herein.