



Find all of your laboratory and workplace safety supplies at Safety Emporium!
 OSHA |
 Glossary Index |
 Palpitation |
| MSDS Topics |
Free Sites | FAQ's | Regulations | Glossary | Software | Suppliers |
| Books | Forum | Poll | Fun stuff | Quiz | Store | |
| Understand your MSDS with the MS-Demystifier | Search ALL our MSDS info | |||||

Be sure you're in compliance with DOT placards and labels from Safety Emporium.
Oxidation can be defined in several different ways. The simplest and most rigorous of these is "the loss of electrons from an atom, compound or molecule."
In general use, the term is generally applied to a chemical reaction of a substance with oxygen (O2) or an oxygen-containing material which adds oxygen atom(s) to the compound being oxidized.
Either way, you can not create energy from nothing. Whenever something is oxidized, something else must undergo the opposite (gain of electrons), reduction.
A species that causes oxidation is called an oxidant, oxidizer, or oxidizing agent.
A species that causes reduction is called a reductant or reducing agent.
Oxidation and reduction are easier to explain using oxidation states (also called oxidation numbers). Oxidation states are assigned to atoms in a molecule or compound using a general set of rules. Take a look at this tutorial to see the rules and test yourself.
A simple example of an oxidation-reduction reaction (remember you can't have one without the other) is the reaction of hydrogen gas with oxygen gas to form water:
2 H2 + O2 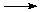 2 H2
2 H2

We have all kinds of safety wall signs at Safety Emporium.
In this reaction, oxygen oxidizes hydrogen from H2 in the zero oxidation state to H+ in the +1 oxidation state. Oxygen, in turn, must be reduced from the zero oxidation state to O2- in the -2 oxidation state. This particular reaction also produces a large amount of heat energy (exothermic, can you say Hindenberg?).
By definition, during an oxidation-reduction reaction the oxidizer (oxidant) is always reduced and the reducing agent (reductant) is always oxidized.
Another example of the tremendous amount of heat that can be generated by an oxidation-reduction reaction is the thermite reaction (link includes video). Here, iron oxide (rust, containing Fe3+) is reduced to iron metal (Fe0) and the aluminum (Al0) is oxidized to aluminum oxide (containing Al3+):
Fe2O3 (solid) + 2 Al (solid) 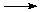 2 Fe (liquid) + 2 Al2O3 (solid) + lots of heat.
2 Fe (liquid) + 2 Al2O3 (solid) + lots of heat.
This reaction generates so much heat that the iron metal product is molten. In fact, this reaction can be used to weld pipes underwater.
This series of terms usually appears on a Safety Data Sheet to warn of very high chemical reactivity and/or the potential for a serious accident if the material comes into contact with incompatibles. This information will be found in Section 10 (stability and reactivity) of the SDS. It may found in Section 6 (accidental release measures) in the context of a spill cleanup procedure or possibly Section 7 (handling and storage) with respect to properly segregating chemicals during storage.
Oxidation reactions are usually very exothermic. Therefore, if a compound says "OXIDIZER", this means that it can cause other materials to combust more readily (or upon contact!) or make fires burn more fiercely. This property may be explicitly noted in Section 5 (fire-fighting measures) of the SDS.
Always store oxidizers away from flammable or combustible materials as well as sources of heat, flame or sparks. Be sure to examine the SDS and label carefully to determine which materials are incompatible.

Flammable storage safety cabinets from Safety Emporium can help you with proper chemical segregation and storage.
See also: air, exothermic, incompatible.
Additional definitions from Google and OneLook.
Entry last updated: Saturday, January 7, 2023. This page is copyright 2000-2025 by ILPI. Unauthorized duplication or posting on other web sites is expressly prohibited. Send suggestions, comments, and new entry desires (include the URL if applicable) to us by email.
Disclaimer: The information contained herein is believed to be true and accurate, however ILPI makes no guarantees concerning the veracity of any statement. Use of any information on this page is at the reader's own risk. ILPI strongly encourages the reader to consult the appropriate local, state and federal agencies concerning the matters discussed herein.