
Sigma-bond metathesis is easier to explain with a drawing than with words:
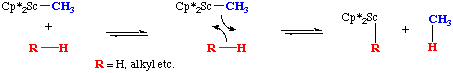
As shown in the drawing, a sigma-bonded ligand is replaced through reaction with the sigma bond of an incoming ligand. Notice that this four-center mechanism does not involve a change in oxidation state. Sigma-bond metathesis is a common reaction with early transition metals that are in their highest oxidation state. Such complexes can not undergo an exchange of ligand by a pathway involving an oxidative addition followed by a reductive elimination. For example, oxidation addition in the example shown above would require the unlikely/impossible Sc5+ intermediate,Cp*2ScMeHR.
Sigma bond metathesis is generally confined to those systems where oxidative addition is not a viable pathway. This makes sigma bond metathesis a common reaction in lanthanide complexes as the lanthanides generally have only one common oxidation state (3+). Sigma bond metathesis plays a critical role in certain olefin polymerization reactions and is frequently encountered in reactions involving dihydrogen or silanes.