
As the name suggests, an insertion reaction involves the formal insertion of one ligand (usually unsaturated) into another metal-ligand bond on the same complex. The generic reaction is shown below, where U = an unsaturated ligand:
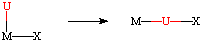
U = CO, C2H4, C2R2, NO, CR2, CNR, RCN, O2, CO2
X = H, alkyl, aryl, OR, NR2
The net result is a decrease in the coordination number by one, along with the formation of a new U-X bond. The reverse of this reaction is generally called a deinsertion. When the deinserting group is an olefin, this has the special name of beta-hydride elimination.
For a generic insertion of CO into a metal alkyl bond, one can envision two mechanistic extremes, one in which the methyl migrates to the carbonyl and a second in which the carbonyl moves and inserts into the metal-methyl bond. Either way, this generates an open coordination site denoted here as the small box:
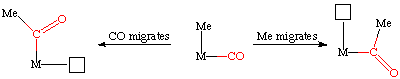
This subtle but important difference was studied by Calderazzo (see Ang. Chem. Int. Ed. Eng. 1977, 16, 299 for his classic IR spectroscopy study) who examined the reverse reaction, deinsertion of CO from a metal acyl complex. By the Principle of Microscopic Reversibility, the insertion and deinsertion must follow the same mechanistic route, only in different directions.
By labeling one of the carbonyls cis to the acetyl group with 13CO, one can differentiate the two possible mechanisms. If the CO moves during the deinsertion, then it can only move to a cis position, displacing another CO in the process. As there are four cis CO's and only one of them is labeled with 13CO, then we would expect to remove the labeled carbonyl 25% of the time.
If the methyl group moves, it can also displace the 13CO 25% of the time. However, if it moves into one of the other three cis positions, it can do so in a cis and trans fashion with respect to the 13CO, something that can be detected spectroscopically:
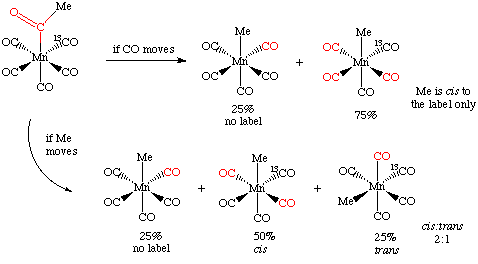
The experimental result, a 2:1 cis:trans ratio, is that the methyl group moves. For this reason, insertion reactions are sometimes called migratory insertion reactions. Of course, not all insertions have to take place by this mechanism.
For example, here's a case by Flood (Organometallics 1983, 2, 1590) in which both mechanisms appear to operate! This terrific example uses an optically resolved transition metal complex to determine which group moved. The mechanism differs depending on the solvent used for the reaction:
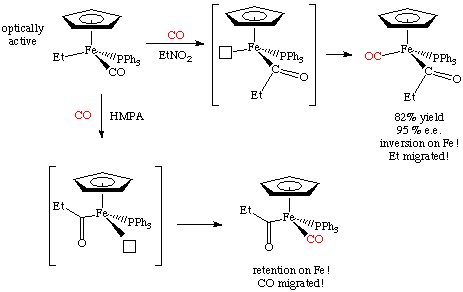
The migratory aptitude of alkyl groups follows the order Me > Ph > benzyl. Double or greater insertions of carbon monoxide are exceedingly rare although a few good examples are known (see Organometallics 1992, 11, 2534 for one example):
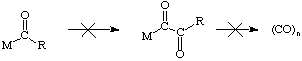
In contrast, multiple insertions of other substrates such as olefins are common and very important. Alternating insertions of CO and ethylene to afford copolymers have also been reported.
Insertion of CO into a metal hydride is also exceedingly rare. That's because transition metal formyl complexes are metastable, decomposing to metal hydrides unless there is an unusual stabilization of the carbonyl oxygen by an oxophilic metal center such as Th (J. Am. Chem. Soc. 1981, 103, 6959):
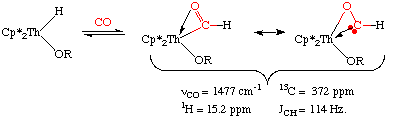
Notice the reduced C=O bond order in the above species as well as the 13 resonance of the formyl carbon at 372 ppm. Together, both these data suggest an oxycarbene-like character with a JCH of 114 Hz.
Solvent can play a role in migratory insertions. This was studied first by Basolo who suggested that direct attack of solvent at the metal center could induce insertion. This was later confirmed by Bergman, who studied the steric effect of solvent on insertion in a Cp-Mo system (J. Am. Chem. Soc. 1981 103, 7028).
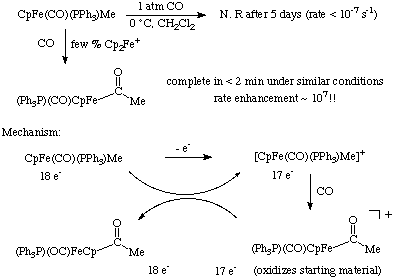
CO insertions can also be induced by oxidation of the metal center. The reactions below highlight a case of electron transfer catalysis of the insertion. Notice the extreme reactivity of the 17 electron intermediate.
The generic example of an olefin insertion is into a metal-alkyl or metal-hydride bond:

The reverse process is called a beta-hyride elimination.
The transition state for this process is essentially an agostic interaction of the beta hydrogen of the nascent alkyl:
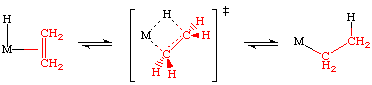
Evidence for a transition state of this sort is supported by the X-ray and neutron structures. Consider the following two examples:
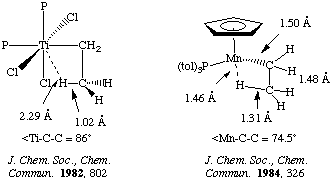
Olefin insertion into metal hydrides is usually an equilibrium process. For very electron-withdrawing olefins the reaction may lie completely to the right. There are relatively few examples of a single olefin insertion into a metal alkyl, however this forms the basis for a number of catalytically important processes such as olefin polymerization! Thermodynamically, the reaction is favored (unlike the formyl case discussed above under CO insertions), and this experimental rarity can be attributed to kinetics.
The rates of olefin insertions into metal hydrides or alkyls are quite fast. For example, Doherty and Bercaw (J. Am. Chem. Soc. 1985, 107, 2670) had to use spin saturation transfer (also called magnetization transfer) techniques to study one such insertion.