



Find all of your laboratory and workplace safety supplies at Safety Emporium!
 PEL |
 Glossary Index |
 pH |
| MSDS Topics |
Free Sites | FAQ's | Regulations | Glossary | Software | Suppliers |
| Books | Forum | Poll | Fun stuff | Quiz | Store | |
| Understand your MSDS with the MS-Demystifier | Search ALL our MSDS info | |||||
A peroxide is a chemical substance that contains a peroxo unit, one that has a chemical formula of O22-. The most familiar example of a peroxide is hydrogen peroxide, shown below on the left with the peroxo unit in red. In lab slang, the term "peroxide" is sometimes used as shorthand for hydrogen peroxide.
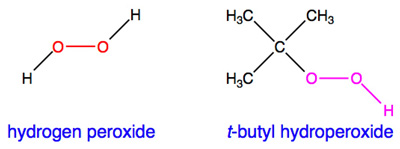
Hydroperoxides contain the O-O-H- unit. Hydrogen peroxide is therefore also an example of a hydroperoxide. One of the most commonly used hydroperoxides is called t-butyl hydroperoxide (or tert-butyl hydroperoxide), shown above on the right.
DANGER!!! DANGER!!! DANGER!!!
Peroxides and hydroperoxides are highly reactive materials and some are extremely shock-sensitive explosives. Peroxides can form readily in certain organic materials, especially ethers. Simply moving or just screwing the cap off a bottle that is contaminated with peroxides can lead to an explosion, injury and/or death. Read all of the following information carefully!
Here are actual pictures of dangerous peroxide crystals:


Thanks to David E. Blair of Heritage ETS for the pictures. A web search for peroxide pictures will provide more examples.
Peroxides can occur in virtually any kind of organic chemical, however, certain chemicals are particularly prone to peroxide formation and pose special hazards. Here is a list of some of the most common peroxide-forming chemicals drawn mostly from Control of Peroxidizable Compounds in J. Chem. Ed 1970, 47(3), A176 (subscription required to view on-line). This list is not comprehensive. Other materials that are chemically similar (alkenes, ethers, allylic and propargylic species) generally have similar risks.
Note: Some inorganic peroxides are generally OK to handle, but pose serious incompatibility issues when used with organic chemicals. If you use any peroxide that is sold in pure form, read the Safety Data Sheet very carefully before use.
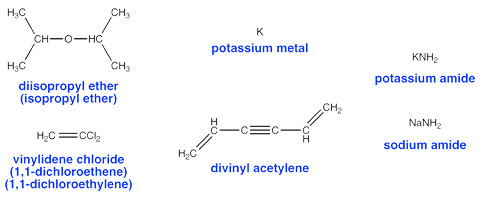
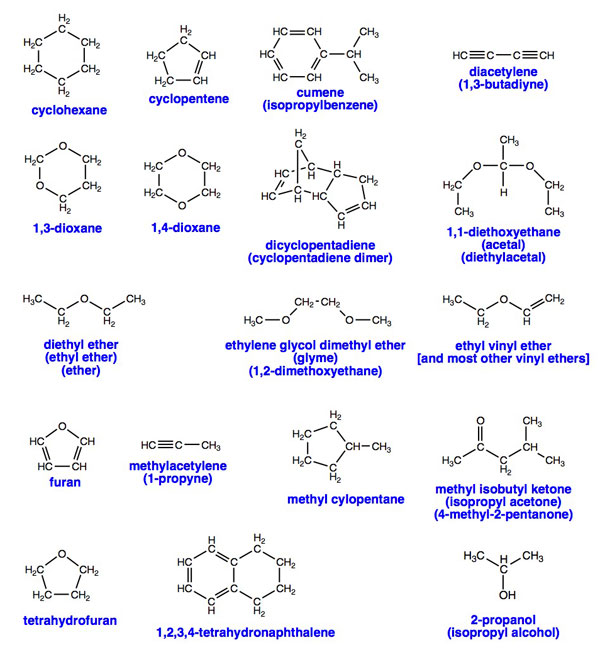
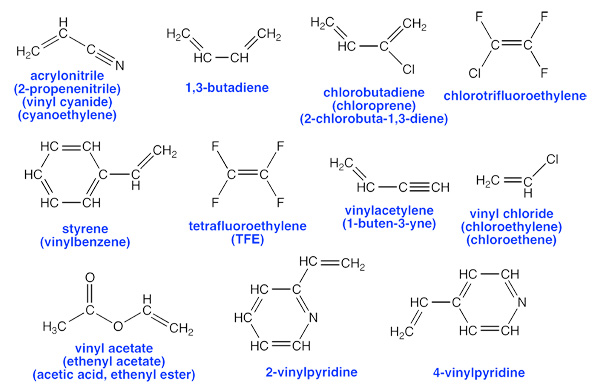

Pick a good database program to maintain your chemical inventory list. Ideally, you'd like to have a special field or checkbox for peroxide-forming materials.

To see what these crystals can look like in a severe case visit this archived page from Reactive Hazards Reduction Inc. and be sure to read about the resulting detonations!
For another amazing peroxide story see Deactivating a Chemical Bomb, a 1998 story about an unstabilized cylinder of 1,3-butadiene, courtesy of the Internet Wayback Machine.
If you encounter such a bottle, contact your Environmental Health and Safety office. If your organization does not have someone equipped to deal with or assess this hazard, call your local police department bomb squad (no kidding). DO NOT TOUCH OR MOVE THE SUSPECT BOTTLE YOURSELF FOR ANY REASON.
Important warning - Any test method can give dangerously false low readings if:
See the links to the DOE Office of Health Safety and Security under Further Reading for several examples of where false low readings have lead to explosions.
There are a variety of methods suitable for the detection of peroxides. Methods C and D are the most commonly used for ethers. Method A is a best bet for anyone who has little laboratory experience. In all cases run a blank sample (one you know doesn't form peroxides such as n-hexane) so you know what a negative result looks like. If possible, also run a blank sample that you have spiked with some hydrogen peroxide so you know what a positive result looks like.
Methods for detecting peroxides include:
Peroxides will oxidize the colorless iodide anion, I-, to elemental iodine, I2, which gives purple or brownish solutions depending on the solvent. Purple, brown or purple-brownish colors indicate relatively high concentrations of peroxides and yellowish colors indicate low concentrations.
You can prepare your own starch-iodide test strips by dunking strips of filter paper into your starch iodide paper and allowing them to dry. Store these away from light in a dry place. Be sure to test these against a dilute hydrogen peroxide, H2O2, solution so you know they work correctly.
Chemists will have to deal with peroxides at some point in their careers. For low levels of peroxides, there are steps you can take to remove or eliminate these contaminants, but you should only do so after verifying the peroxide levels. If you see visible white crystals in the container or around the cap then STOP. This is a bomb squad job.
Caution: use the following accepted procedures at your own risk. If you have no experience in dealing with such situations do NOT try any of these corrective procedures. NEVER ATTEMPT TO DEAL WIth WHITE PEROXIDE CRYSTAL DEPOSITS YOURSELF. Call your local police bomb squad (if you do not have one, your state or provincial police will surely have one) to deal with the container.
There are different methods available. Which one you pick depends on knowing the chemical properties of the material and any possible incompatibilities with the materials used to treat the peroxides. Either of the first two methods would be a good choice for diethyl ether, for example.
Note that destruction of high level of peroxides is a procedure fraught with peril and risk of bodily injury, including death. See the links to the DOE Office of Health Safety and Security under Further Reading for several examples where attempted treatment of peroxides led to unexpected outcomes.
Peroxide formation in organic materials is usually initiated by a free radical, a molecule with an unpaired electron. Free radicals are hard to avoid as they are continually formed by exposure to light or heat. For this reason, chemicals that are likely to form peroxides often contain an inhibitor that will consume free radicals as they are formed. A common inhibitor is BHT (butylated hydroxytoluene, also used as a food preservative).
In the example below we'll use R. to represent a generic alkyl radical (the dot represents the unpaired electron). In the case of diisopropyl ether, the free radical abstracts a hydrogen atom from a carbon next to the ether oxygen atom. This forms an RH molecule (called an alkane) and gives the ether molecule an unpaired electron. The ether is now the radical!
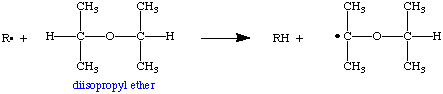
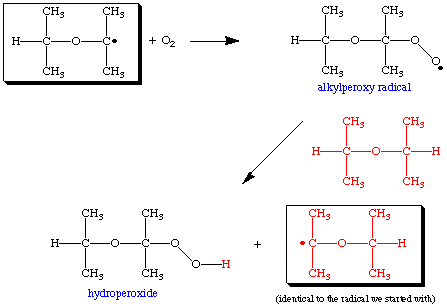
The ether radical can react with atmospheric oxygen (O2) to form an alkylperoxy radical, which in turn can react with a second molecule of ether to form a hydroperoxide and generate an ether radical identical to the one we started with. This new radical can also react with oxygen to form additional hydroperoxide and generate an identical radical. This process can go on virtually forever, so this is sometimes called a catalytic chain reaction or autooxidation.
From here, any of several steps can happen. For example, the alkylperoxy radical could react with an ether radical directly to form a dialkyl peroxide:
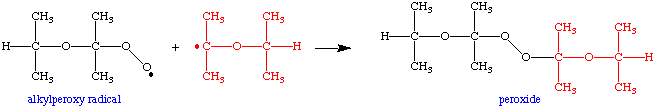
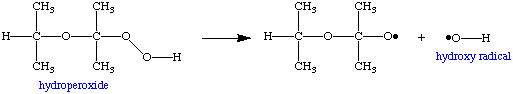
Likewise, at slightly elevated temperatures (ballpark: 70 degrees C), the hydroperoxide molecule can decompose to form two new radicals, both of which can go on to catalyze the formation of additional hydroperoxide, and generate even more heat. The hotter things get, the faster the decomposition and the rate of radical formation from hydroperoxide. This can obviously self-accelerate and lead to an explosion. Such situations are called autocatalytic reactions
Read your Safety Data Sheet and be on the lookout for peroxide-formers. Read this ENTIRE document (OK, you can skip the chemistry of formation if you wish). DO NOT let your guard down when working with chemicals that can form peroxides. These can pose an incredible health and safety danger, but such danger can be easily avoided with a regular monitoring program and awareness of the potential hazards.
See also: decomposition, ether, explosive.