



Find all of your laboratory and workplace safety supplies at Safety Emporium!
 Erythema |
 Glossary Index |
 Evaporation Rate |
| MSDS Topics |
Free Sites | FAQ's | Regulations | Glossary | Software | Suppliers |
| Books | Forum | Poll | Fun stuff | Quiz | Store | |
| Understand your MSDS with the MS-Demystifier | Search ALL our MSDS info | |||||
An ether molecule has an oxygen atom connected to two alkyl (see hydrocarbon) units through carbon-oxygen single bonds. The two alkyl groups can be the same or different:
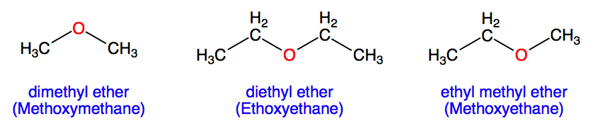
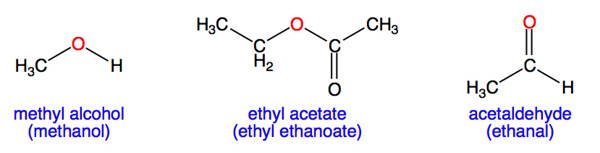
The following are NOT examples of ethers because the oxygen atom is not connected to two different carbon atoms via single bonds or because the attached group is not an alkyl:
Ether is also used as shorthand to refer to one of the most common ethers, diethyl ether, shown above.

Get warning labels and DOT shipping placards at Safety Emporium.
The term "petroleum ether" is an old term used to refer to a mixture of hydrocarbons with a specific boiling point range. In other words, there is no ether in petroleum ether! Synonyms for petrolem ether include naphtha, petroleum benzin, benzin, and the lab slang, "pet ether". The petroleum ether most commonly used in laboratories has a boiling point range of roughly 30 to 60 °C.
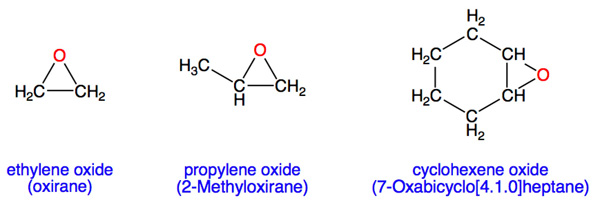
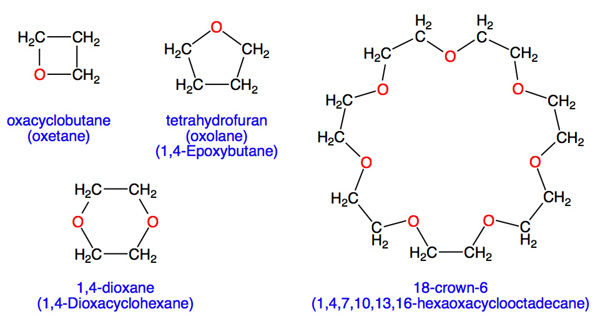
In many ethers, the atoms are arranged in a closed ring:

Laboratory operations are a breeze with Ohaus analytical balances from Safety Emporium.
Ethers and mixtures that contain ethers will always have a Safety Data Sheet that you should read thoroughly before use.
Again, the risk of peroxide formation is particularly high with ethers. Be sure to read about peroxides so you know how to detect, avoid and/or deal with them should you encounter them. Many ethers (such as diethyl ether) are highly volatile and flammable. Diethyl ether does not require a flame or spark to ignite and can be ignited by static electricity or a hot plate.
Many ethers, particularly the crowns, are eye and skin irritants and/or toxic. The halogenated ethers such as bis(chloromethyl) ether and chloromethyl methyl ether are known human carcinogens.
Always read the SDS before using an ether so you know what safety precautions to take. Use appropriate engineering controls, wear appropriate personal protective equipment (PPE), have good ventilation, and avoid heat or ignition sources.

Get your SDS binders, centers and more from Safety Emporium.
Additional definitions from Google and OneLook.
Entry last updated: Sunday, July 24, 2022. This page is copyright 2000-2025 by ILPI. Unauthorized duplication or posting on other web sites is expressly prohibited. Send suggestions, comments, and new entry desires (include the URL if applicable) to us by email.
Disclaimer: The information contained herein is believed to be true and accurate, however ILPI makes no guarantees concerning the veracity of any statement. Use of any information on this page is at the reader's own risk. ILPI strongly encourages the reader to consult the appropriate local, state and federal agencies concerning the matters discussed herein.