



Find all of your laboratory and workplace safety supplies at Safety Emporium!
 Incompatible |
 Glossary Index |
 Inflammation |
| MSDS Topics |
Free Sites | FAQ's | Regulations | Glossary | Software | Suppliers |
| Books | Forum | Poll | Fun stuff | Quiz | Store | |
| Understand your MSDS with the MS-Demystifier | Search ALL our MSDS info | |||||
An inert chemical substance is one that is not generally reactive. This is a synonym for "inactive" with respect to chemical reactions.
Inert has a non-chemical meaning of being unable to move or resist movement; for example, "the accident victim was laying on the ground, inert."
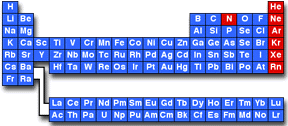
In the periodic table of the elements shown below, the inert elements are shown in red. The noble gases, the last column of the table, include helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). Nitrogen (which, in the elemental form, occurs as N2 gas) is also considered inert although it forms a wide variety of chemical compounds.

Lab hazards require special extinguishing agents such as Lith-x, available at Safety Emporium.
These elements are unreactive because they are very stable in their naturally occurring forms. While some of these can be made to react chemically, their compounds are usually not very stable (except for nitrogen). The term inert atmosphere is usually used to denote a nitrogen or argon atmosphere in a container.
Chemical compounds can also be considered inert. For example, poly(tetrafluoroethylene), better known by the DuPont tradename Teflon™, does not react with most substances. Likewise, sand, SiO2, is generally unreactive.
We can also use the term to describe reactivity (or lack thereof) towards particular substances. For example, mercury reacts with aluminum metal (which is one reason why it is illegal to transport liquid mercury by aircraft) but is inert towards iron metal. Carbon dioxide is inert to many chemical reactions, but is incompatible (and can react violently) with alkali metals such as sodium and potassium. Using a carbon dioxide fire extinguisher on a magnesium fire would be a VERY bad idea.
Likewise, one may see the term "inert" used on pharmaceutical or pesticide labels to indicate components that are not active ingredients/components of the mixture. For example, pills are held together with binders that simply dissolve to release the medicine inside the pill. As the binder does not have any biological effect it may be referred to as a biologically inert ingredient.
While chemical inertness and biological inertness are often the same, sometimes a substance can be one and not the other. For example, while xenon does not chemically react in the human body, it nonetheless has biological effects that have been exploited for anesthesia as well as improvement of tissue damage caused by inadequate blood supply (ischemia)
Inert materials are good choices for chemical containers. For example, acid waste should not be stored in metal drums because these will quickly corrode. However, glass or polyethylene containers are inert to most acids.
If a chemical spill occurs, one may need to clean up the spill by using an inert absorbing material such as vermiculite or sand. The Safety Data Sheet will usually recommend a specific material, but not always. Assuming your SDS was created using the format required under HCS 2012, spill cleanup information will be found under Section 6 (accidental release measures). But be sure to read the rest of the sheet as well, because it's important that you know the physical properties of the material, the health hazards, incompatibilities etc.

Safety Emporium has a great lineup of gas cylinder signs, storage racks, clamps and more.
If the SDS is not clear, remember that you can phone the manufacturer at the phone number listed on the SDS. If you are using a spill kit, see if it came with a guide or instructions.
See also: asphyxiant, corrosive, flammable.
Additional definitions from Google and OneLook.
Entry last updated: Monday, January 2, 2023. This page is copyright 2000-2025 by ILPI. Unauthorized duplication or posting on other web sites is expressly prohibited. Send suggestions, comments, and new entry desires (include the URL if applicable) to us by email.
Disclaimer: The information contained herein is believed to be true and accurate, however ILPI makes no guarantees concerning the veracity of any statement. Use of any information on this page is at the reader's own risk. ILPI strongly encourages the reader to consult the appropriate local, state and federal agencies concerning the matters discussed herein.