



Find all of your laboratory and workplace safety supplies at Safety Emporium!
 Flash point |
 Glossary Index |
 Fume |
| MSDS Topics |
Free Sites | FAQ's | Regulations | Glossary | Software | Suppliers |
| Books | Forum | Poll | Fun stuff | Quiz | Store | |
| Understand your MSDS with the MS-Demystifier | Search ALL our MSDS info | |||||

Get your freeze-protected fire fighting foam concentrates and other extinguishing agents at Safety Emporium.
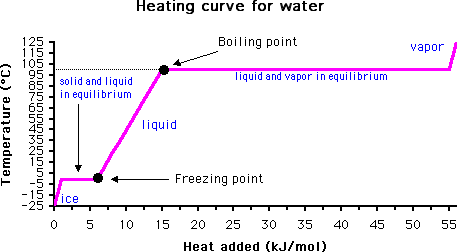
Freezing point is the temperature at which a liquid becomes a solid at normal atmospheric pressure. Alternatively, a melting point is the temperature at which a solid becomes a liquid at normal atmospheric pressure.
A more specific definition of freezing point is the temperature at which solid and liquid phases coexist in equilibrium. The normal freezing point is the temperature at which a substance melts (or freezes) at one atmosphere (760 torr = 760 mm Hg = 14.7 psi = 101.3 kPa) of pressure.
Melting points can be determined by visual inspection or by monitoring the temperature of the liquid with time. When the freezing point is reached, the temperature of the solution will no longer drop until all the liquid has frozen.
Melting points may be lowered (depressed) by the addition of a soluble material to the solution. The degree of lowering depends on the solvent and is easily predicted using a simple equation that is beyond the scope of this resource (see below).
Pay close attention to the temperature units when reporting or reading a melting/freezing point. Most scientific literature uses the Celsius scale whereas (in the US), most consumer products use the Fahrenheit scale. The two are not interchangeable.

Safety Emporium carries freeze-protected eye/face washes and safety station.
Per Appendix D of 29 CFR 1910.1200, the OSHA Hazard Communication Standard (HCS), the melting/freezing point is a required piece of data on a Safety Data Sheet. Assuming the sheet was issued since 2015 and is GHS-compliant, you will find this data listed in section 9 of the SDS. Prior to HCS 2012, there was no legally required format for (M)SDS's and this piece of data, while still required, could appear anywhere in the document.
HCS 2012 does not specify temperature units. Typically, you will find both Fahrenheit and Celsius on an SDS, but this is not always be the case, particularly for laboratory chemicals, so be certain that you know which scale is being used!
The freezing/melting point is an important material property to know and the SDS is your primary resource for this information. Some materials must be prevented from freezing to protect their integrity or the product packaging. Likewise, most (but not all) materials expand when heated above their melting point and may overflow or burst their container. Likewise, aqueous solutions tend to expand when they freeze which can also burst the container. Section 7 (handling and storage) of the SDS will note if there are any (temperature-related) considerations for safe storage of the material.
See also: Boiling point, Vapor pressure.
Additional definitions from Google and OneLook.
Entry last updated: Wednesday, December 28, 2022. This page is copyright 2000-2025 by ILPI. Unauthorized duplication or posting on other web sites is expressly prohibited. Send suggestions, comments, and new entry desires (include the URL if applicable) to us by email.
Disclaimer: The information contained herein is believed to be true and accurate, however ILPI makes no guarantees concerning the veracity of any statement. Use of any information on this page is at the reader's own risk. ILPI strongly encourages the reader to consult the appropriate local, state and federal agencies concerning the matters discussed herein.