



Find all of your laboratory and workplace safety supplies at Safety Emporium!
 BOD |
 Glossary Index |
 Bradycardia |
| MSDS Topics |
Free Sites | FAQ's | Regulations | Glossary | Software | Suppliers |
| Books | Forum | Poll | Fun stuff | Quiz | Store | |
| Understand your MSDS with the MS-Demystifier | Search ALL our MSDS info | |||||
How can one determine the temperature at which water boils? After all, bubbles start to appear in water well below the known boiling point of 100 degrees C (212 °F).

Safety Emporium has all kinds of lab equipment such as stirring hot plates.
The answer lies in monitoring the temperature of the material with time. When the boiling point is reached, the temperature will not rise again until all of the liquid has evaporated. This is due to the high heat capacity of water (it takes much more energy to convert water from liquid to gas than it does to raise the temperature of liquid water).
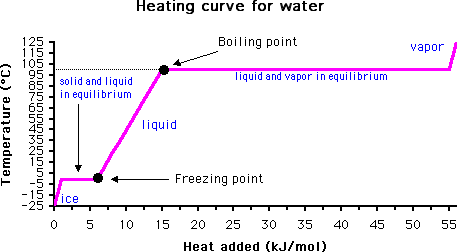
Of course, if water is heated under pressure this may raise the boiling point above its normal boiling point of 100 degrees C. Likewise, the addition of a solute may also raise the boiling point, a phenomenon called boiling point elevation (see Further Reading below for more information).
Not all substances have a boiling point. Some substances may decompose into other materials when heated instead of boiling. Wood does not boil, and neither does calcium carbonate, which decomposes to calcium oxide and carbon dioxide when heated. Other substances, such as solid carbon dioxide, may sublime to give gases without ever forming a liquid under normal conditions. However, under higher pressure, carbon dioxide will turn to liquid and then boil as the temperature is raised. Therefore, it is a best scientific practice to always report the external pressure when reporting a boiling point.
If applicable and known, the boiling point of a material is required to appear in Section 9 (physical and chemical properties) of the material's Safety Data Sheet.
Knowing the boiling point of a substance is an important consideration for storage. For example, storing a chemical with a boiling point of 50 oC (122 oF) in direct sunlight or next to a boiler could cause the material to completely vaporize and/or result in a fire or explosion.
Items with a low boiling point generally have a high vapor pressure. Containers of such material can build up signicant pressure even when they are below their boiling point. Likewise, low-boiling materials easily produce large amounts of vapor which can be flammable or even explosive.

Reactions run more easily with laboratory heating mantles and temperature controllers from Safety Emporium.
See also: evaporation rate, flash point, freezing point, vapor pressure.
Additional definitions from Google and OneLook.
Entry last updated: Tuesday, April 19, 2022. This page is copyright 2000-2025 by ILPI. Unauthorized duplication or posting on other web sites is expressly prohibited. Send suggestions, comments, and new entry desires (include the URL if applicable) to us by email.
Disclaimer: The information contained herein is believed to be true and accurate, however ILPI makes no guarantees concerning the veracity of any statement. Use of any information on this page is at the reader's own risk. ILPI strongly encourages the reader to consult the appropriate local, state and federal agencies concerning the matters discussed herein.