
|
Safety Emporium for all your lab and safety needs
 The Glassware Gallery
The Glassware Gallery
Powder X-Ray Diffractometers
Powder XRD
Powder Powder X-ray Diffraction (XRD) is one of the primary techniques used by solid state chemists to characterize materials. Powder XRD can provide information about crystalline structure (or lack thereof) in a sample even when the crystallite size is too small for single crystal x-ray diffaction.
The Diffractometer
Shown here is a powder x-ray diffractometer. You can see a recirculating chiller in the back right corner of the room (the blue box). The role of the chiller is to cool the x-ray tube which is contained in the base of the XRD unit.

The diffractometer is enclosed with a leaded Plexiglass shield to minimize exposure to scattered X-rays. Shown below is a closer view inside the shielding.
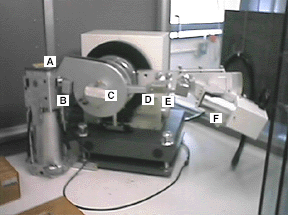
X-rays are produced when a 35 keV electron beam strikes a copper target. The x-rays are emitted horizontally through a shutter (A) on the left, travel through a focusing slit (B) and then impinge on the sample (C, which can not be seen in this photo because a safety cover is in place). The emerging beam travels through two more slits (D and E) as well as a thin nickel foil (to remove most of the k-beta and white radiation). Finally, the x-rays impinge on the detector (F).
The arm holding the detector assembly (D-F) pivots during the run. If data were collected from 0 to 90 degrees 2-theta, then the detector assembly would be positioned directly above the sample (C) at the end of the run.

Get your radiation safety signs and labels at Safety Emporium.
The XRD Controller
The diffractometer control and data collection is handled through a dedicated PC. Data collection times vary depending on the nature of the data required. For example, a routine XRD spectrum can be collected in about 40 minutes, while one suitable for a Rietveld refinement might require 16 hours.
Older instruments simply record the output file to a strip chart recorder. Modern instruments can store and manipulate the pattern and have many powerful features. One of the most useful is the ability to match an unknown material against a database of 70,000+ known phases.
Sample Preparation
Sample requirements for powder XRD vary with the nature of the material. A typical sample holder is a 2 mm thick aluminum plate with a 20 mm square hole in the center. For materials that diffract strongly (many inorganic materials), Scotch tape is placed over the hole with the sticky side up. About 10-20 mg of the material of interest is then spread on the tape and smoothed flat. The tape is primarily amorphous and so does not generally interfere with the pattern being collected.
For materials that diffract less strongly such as organic molecular crystals, more sample (100-200 mg) must be used. One way of doing this is to tape or glue a microscope slide to the back side of the sample holder. The depression in the holder is then filled with sample and smoothed flat. Air sensitive samples are handled in special holders that have an airtight seal and Mylar windows. Bruker has a product sheet titled Specimen Holders for X-ray Diffraction that has pictures of these and other types of sample holders. Mylar windows are available from various vendors including Chemplex
XRD samples should be well-ground in a mortar and pestle or a ball mill. This creates a uniform particle size and ensures that all possible crystallite orientations are present in the sample. A special problem that can arise in sample preparation is called preferred orientation which usually occurs with rod or plate-like crystals. For example, plate-like crystals tend to lie flat on the sample holder; very few will have a perpendicular orientation. As there is no longer a random orientation of crystallites some of the x-ray reflections that would be expected are unusually weak or missing altogether.
Preferred orientation can sometimes be avoided by slurrying the material of interest in a volatile organic solvent such as acetone or pentane. This slurry is poured into a sample holder that has a microscope slide taped to the back. With a little luck, the solvent evaporates before the slurried crystals have an opportunity to re-orient themselves. Preferred orientation can also be addressed using Rietveld software, but this is not always possible or reliable.
Further Reading
- The International Centre for Diffraction Data collects and publishes diffraction files. Lots of info and a free downloadable demo of their database.
- Download an X-ray diffraction simulator (Mac only) that outputs XRD patterns based on an input unit cell.
- A brief primer on powder XRD at the U.S. Geological Survey.
- Introduction to X-ray diffraction at Matter Online. Includes info on indexing patterns and more.
