



Find all of your laboratory and workplace safety supplies at Safety Emporium!
 Anhydride |
 Glossary Index |
 Anorexia |
| MSDS Topics |
Free Sites | FAQ's | Regulations | Glossary | Software | Suppliers |
| Books | Forum | Poll | Fun stuff | Quiz | Store | |
| Understand your MSDS with the MS-Demystifier | Search ALL our MSDS info | |||||
An anhydrous material does not contain any water (H2O) molecules.
Many substances occur naturally as hydrates, compounds that have a specific number of water molecules (H2O) attached to them. This water can often be removed by heating and/or vacuum to give the anhydrous material.

Get drying apparatus and other organic laboratory equipment and glassware from Safety Emporium!
Anhydrous materials can absorb water from their surroundings and find use as desiccants (drying agents). Examples include the packets of silica gel one finds in some consumer goods, as well as dehumidifying sachets used in clothes closets. See our entry on hygroscopic for more details.
Anhydrous materials differ from anhydrides. Anhydrides react irreversibly with the oxygen-hydrogen bonds of water to form new compounds whereas anhydrous compounds simply form reversible adducts with water molecules.
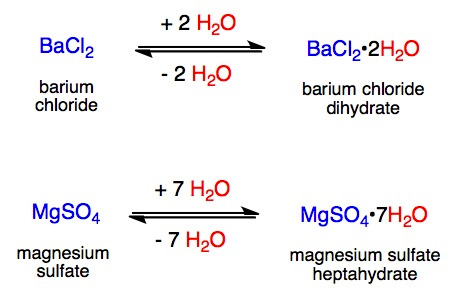
The hydrated forms of the compounds shown below can be converted into the anhydrous forms by heating. This provides a convenient way of recycling (regenerating or reactivating) the drying agent. In each example below, note the double arrows between the products and reactants that indicate a reversible chemical reaction.
A commonly used chemical is anhydrous ammonia (NH3) which is ammonia in its pure form and has very different properties and uses than its aqueous form; see our amine entry for more details.
Some anhydrous materials are considered water reactive materials, meaning that they could release a large amount of heat possibly leading to a pressure or chemical explosion. Water reactive materials will note this in Section 10 (stability and reactivity) of the Safety Data Sheet. Use such materials only with proper precautions and training and pay very careful attention to the firefighting procedures listed in Section 5 of the SDS.
See also: anhydride, hygroscopic.
Additional definitions from Google and OneLook.
Entry last updated: Tuesday, February 22, 2022. This page is copyright 2000-2025 by ILPI. Unauthorized duplication or posting on other web sites is expressly prohibited. Send suggestions, comments, and new entry desires (include the URL if applicable) to us by email.
Disclaimer: The information contained herein is believed to be true and accurate, however ILPI makes no guarantees concerning the veracity of any statement. Use of any information on this page is at the reader's own risk. ILPI strongly encourages the reader to consult the appropriate local, state and federal agencies concerning the matters discussed herein.