ZnS occurs in two common polytypes, zincblende (also called sphalerite) and wurtzite. The two types have these features in common:
- a 1:1 stoichiometry of Zn:S
- a coordination of 4 for each ion (4:4 coordination)
- tetrahedral coordination
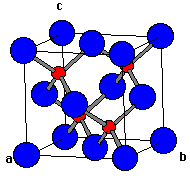
Zincblende/sphalerite is based on a fcc lattice of anions whereas wurtzite is derived from an hcp array of anions. In both structures, the cations occupy one of the two types of tetrahedral holes present. In either structure, the nearest neighbor connections are similar, but the distances and angles to further neighbors differs. Zincblende has 4 asymmetric units in its unit cell whereas wurtzite has 2.
To recap, zincblende is best thought of as a face-centered cubic array of anions cations occupying one half of the tetrahedral holes. Each ion is 4-coordinate and has local tetrahedral geometry. Unlike wurtzite, zincblende is its own antitype -- you can switch the anion and cation positions in the cell and it doesn't matter (as in NaCl). In fact, replacement of both the Zn and S with C gives the diamond structure!
In Figure 1, notice how that only 1/2 of the tetrahedral sites (i.e. four of the eight octants of the cube) are occupied by Zn2+ (red).
 |
 |
| Fig. 1 A single unit cell of zincblende |
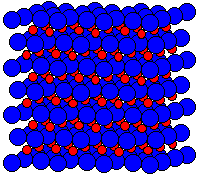
Fig. 2 A 3x3x3 lattice of zincblende |
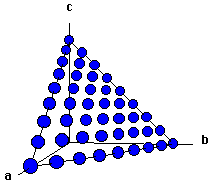
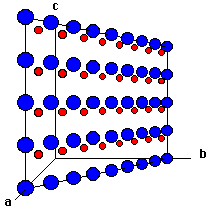
Shown below are two crystallographic planes in zincblende. Notice that the (111) plane is identical to the (111) in NaCl. That's not too surprising given that the anion lattice is identical. Unlike NaCl, the (100) plane of zincblende contains only anions. Look at Figure 4 -- can you see how this cuts through the unit cell in Figure 1?
 |
 |
| Fig. 3 The (111) plane of zincblende. |
Fig. 4 The (110) plane of zincblende. |
Here is a polyhedral representation of the zincblende structure. We could have drawn tetrahedra around either of the two atoms. Notice that all of the tetrahedra point in the same directions (recall that we have T- and T+ sites in fcc lattices...).
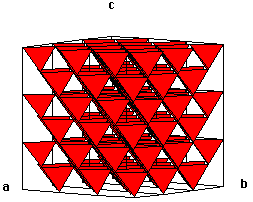
Chime models
Shown here is a Chime model you can rotate and manipulate. (I need to update these to jmol; just haven't found the time yet) Notice how you can see the diamond-like lattice if you rotate the models correctly. Also note the non-planar six-membered rings.
| (Sorry, Chime is unavailable) |
(Sorry, Chime is unavailable) |
| Fig. 5 A single unit cell of zincblende. |
Fig. 6 2x2x2 units cells of zincblende. |
[Structure World Home Page]






