
|
Safety Emporium for all your lab and safety needs
Structure World
The Cesium Chloride Structure

General Notes
Structural Information
| CsCl Vital Statistics | |
|---|---|
| Formula | CsCl |
| Crystal System | Cubic |
| Lattice Type | Primitive |
| Space Group | Pm3m, No. 221 |
| Cell Parameters | a = 4.123 Å, Z=1 |
| Atomic Positions | Cl: 0, 0, 0 Cs: 0.5, 0.5, 0.5 (can interchange if desired) |
| Density | 3.99 |
| Melting Point | 646 degrees C |
| Alternate Names | none |
| Isostructural Compounds |
NH4X, TlX (X = halide), CuZn (beta-brass), AgZn, LiHg, MgSr |
Structure
 |
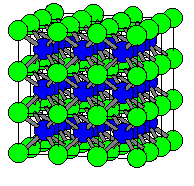 |
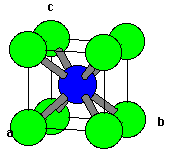
| Fig. 1 A single unit cell of CsCl | Fig. 2 A 3x3x3 lattice of CsCl |
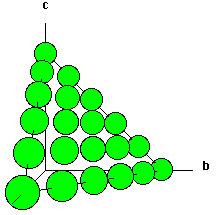
Shown below are two crystallographic planes in CsCl. Notice that the (111) plane is not hexagonally closest packed as it is in the (111) plane of NaCl.
 |
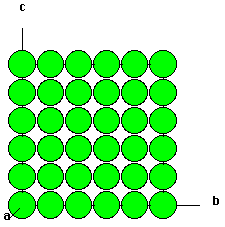 |
| Fig. 3 The (111) plane of CsCl. | Fig. 4 The (100 plane of CsCl. |
- Something that surprises novices is that the (111) plane does not contain the body centered atom. It is important for the beginning student to recognize that the (111) does not cut through 1/2, 1/2, 1/2! Take a look at the models above and below to convince yourself of this very important fact!
Self-Study: Which set of planes contain the body center and the points (0,0,1), (1/2,0,1/2) and (0,1/2,1/2)?
Self-Study: What would a polyhedral representation of this structure look like?
Chime models
| (Sorry, Chime is unavailable) | (Sorry, Chime is unavailable) |
| Fig. 5 A single unit cell of CsCl. | Fig. 6 A 2x2x2 chunk of CsCl lattice. |